Novel molecule shuts down important anti-cancer gene
Our cells have a safety switch that detects when mutations occur that turn normal genes into “cancer genes” and there is a risk for cancer to develop. This mechanism is called senescence, and it arrests the cells so that they can no longer divide. New basic research from BRIC, University of Copenhagen, unveils the function of a novel molecule, MIR31HG, which regulates one of the main players in this safety mechanism, p16INK4A. The results are published in Nature Communications.
Recent research has unveiled a wealth of new molecules that can be exploited to understand and fight cancer.
- MIR31HG belongs to the newly found group molecules called long non-coding RNAs. These molecules exist in large numbers in our cells, but we know very little about their functions and importance in diseases”, says postdoc Marta Montes who has been the main driver of the project.
In the human genome we can find the information that the organism needs to produce all the proteins that a human requires for proper development and function. This information is encoded by the DNA. In order to produce proteins, parts of the DNA first have to be copied into RNA molecules, which form the templates for making proteins. Thanks to very recent techniques, it has been shown that there is also additional information present in the DNA that does not result in the production of proteins – only RNA. This “non-coding” part represents an astonishing 80% of the human genome, and long non-coding RNAs are made from this non-coding part of our genome.
- For many years these long-non coding RNAs were thought to be “noise” with little importance for our development or health. However, many of the long-noncoding RNAs have now been proven to play key roles in important cellular processes as well as in cancer” says professor Anders H. Lund who leads the laboratory at BRIC where the research was performed.
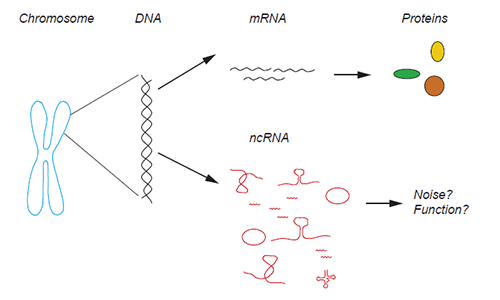
Chromosomes in our cells contain the information to produce proteins. This information is carried in the DNA and needs to be transcribed into messenger RNA (mRNA) before the protein synthesis. However, the majority of the DNA does not encode protein information but is still converted into RNA, this RNA is called non-coding RNA (ncRNA), and it is present in many forms in the cell. Illustration: Marta Montes
MIR31HG suppresses the expression of the anti-cancer gene p16INKA
With cutting-edge techniques, the researchers at BRIC have measured which of the non-coding RNAs are important for senescence and found MIR31HG to be important for the regulation of the well-known anti-cancer gene and cell cycle regulator, p16INK4A.
- We have shown that MIR31HG binds to the p16INK4A gene and facilitates the binding of a group of proteins called Polycomb proteins, which can shut down p16INK4A production, preventing its ability to stop cell division. If we remove MIR31HG from the cells, they enter the process of senescence and stop dividing, because p16INK4A is now expressed”, Marta explains.
The levels of the p16INK4A protein are increased in cells that need to stop dividing, for example, when they sense a cancer threat. A very good example is melanoma, a cancer of the skin. Before becoming malignant, the skin cells sense an activated “cancer gene” BRAF and increase the levels of p16INK4A. They then become senescence and stop dividing. Other mutations that can occur later then may allow the cell to escape this senescence barrier and become malignant melanoma.
- By increasing our knowledge on how anti-cancer genes are regulated, we hope to pave the way for new therapeutic approaches in the treatment of cancer”, Marta Montes finishes.
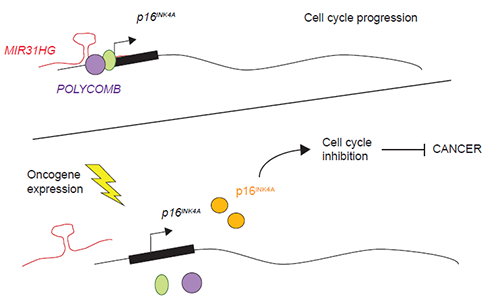
In normal proliferating cells, the lncRNA MIR31HG binds the promoter of the anti-cancer gene p16INK4A together with the Polycomb repressor proteins to prevent p16INK4A expression. In the presence of an oncogenic stimulus MIR31HG and Polycomb proteins are no longer present at p16INK4A promoter allowing p16INK4A expression and therefore, a cell cycle arrest. With this mechanism termed oncogene-induced senescence the cell prevents malignant cells to proliferate. Illustration: Marta Montes
The research is supported by Marie Curie, IEF grant and The Danish Cancer Society.
Contact
Professor Anders H. Lund, BRIC
Phone: +45 3532 5657
E-mail: anders.lund@bric.ku.dk
Postdoc Marta Montes, BRIC
Phone: +45 3533 5039
E-mail: marta.montes@bric.ku.dk
Original paper
The lncRNA MIR31HG regulates p16INK4A expression to modulate senescence. M. Montes, M.M. Nielsen, G. Maglieri, A. Jacobsen, J. Højfeldt, S. Agrawal-Singh, K. Hansen, K. Helin, H.J.G. van de Werken, J.S. Pedersen and A.H. Lund. Nature Communications. April 2015.
