PUBLICATION SPOTLIGHT - PIAS2-mediated blockade of IFN-β signaling: A basis for sporadic Parkinson disease dementia
By Emilie Tresse-Gommeaux, Assistant Professor, Issazadeh-Navikas Group
How would you summarise your research into one sentence?
Our work clearly demonstrates that dysregulation of the immune genes in the brain is a major cause of Parkinson disease and its progression to dementia.
Can you describe briefly what you have explored and how?
Parkinson’s disease is the second most common neurodegenerative disorder of aging and the most common movement disorder. This disease affects 12 000 people in Denmark and 7 to 10 million people world-wide. The etiology of the sporadic form, encompassing 90-95% of cases, is largely unknown. The disease is often progressing to dementia. To determine which molecular pathways were dysregulated in Parkinson disease, we performed bioinformatic analyses from patient brains. We demonstrated that blockage of the type I Interferon (IFN) pathway, a key anti-inflammatory cytokine which is involved in response to viral infection, is the strongest molecular pathway linked to Parkinson disease and its progression to dementia. We recapitulated this in 3 different mouse models using a negative regulator of this pathway, PIAS2, that was identified from the patient study as one of the key proteins linked to the progression of Parkinson disease dementia.
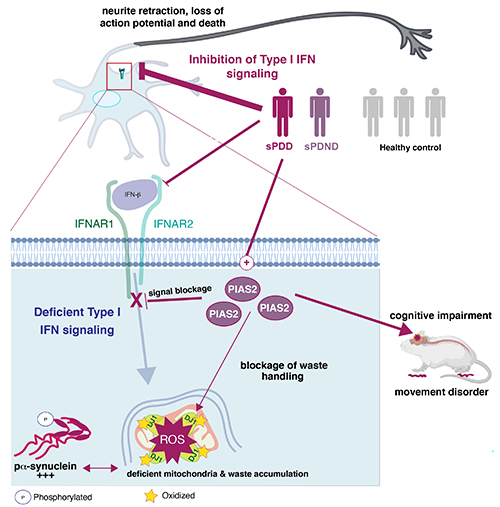
Defects in anti-viral immune response genes, ie. type I IFN signaling via elevated PIAS2 is associated with sporadic PD and particularly its progression to dementia.
Type I IFN signaling pathway is often control bodies response to viral infections. Here we found that blockage of type I IFN signaling pathway, via increased levels of PIAS2 is associated with sporadic PD without dementia (sPDND) and the PD progression to dementia (sPDD). PD patients present overactivation of PIAS2, an inhibitor of type I IFN signaling pathway in the brain. These defects cause neuronal pathology causing neurons to die (neurodegeneration). In particular, type I IFN signaling pathway activates processes which are responsible for damaged protein and mitochondrial (garbage) removal, called autophagy and mitophagy. Upon pathologically high expression of PIAS2 protein and/or blockage of type I IFN, the mitochondria which is normally the powerhouse of the nerve cells and provide highly needed energy, do not function well and this causes accumulation of damaged mitochondrial mass accompanied with elevated oxidized proteins. This further leads to accumulation of other toxic proteins in neurons like pa-synuclein. Reducing or blocking PIAS2 in models of PDD reversed these PDD-like pathologies and clinical manifestations. The figure is an adapted and modified version of Magalhaes & Tresse et al. Molecular Psychiatry. link: https://www.nature.com/articles/s41380-021-01207-w .
What would you say is the novelty in your research?
Our research is the first to demonstrate the role of the type I interferon pathway in regulation of nerve cells’ powerhouse, ie. mitochondria, blockage of this causes Parkinson disease and its progression to dementia.
How does your research relate to the scientific community with respect to what is already known?
We demonstrated previously that mice lacking the type I IFN; interferon beta or its receptor IFNAR develop motor and cognitive dysfunction resembling Parkinson disease dementia (PDD). Here, we show that type I IFN is actually the major dysregulated pathway in PDD patients.
Additionally, study from the familial form of the disease put dysfunction of mitochondria, the power-house of the cell, as major contributor to the pathology. In our current study, we further demonstrate that dysregulation of the type I IFN pathway is leading to defect in damaged mitochondrial clearance causing defects in energy production.
Can you put the work in context with regards to its impact on the non-scientific community?
The first impact is on screening. Mutation/variation of genes involved in the type I IFN pathway could be evaluated for potential roles in familial forms of Parkinson disease. Additionally, the impact of the dysregulated type I IFN pathway, as a result of viral infections, could be evaluated on the neurodegenerative processes in patients.
Finally, and maybe most importantly, as a major contributor to the disease, therapeutic targeting the type I IFN pathway to counteract its blockage is anticipated to have a beneficial impact on the disease, in particular towards preventing dementia.
What are the next stages in this research?
As explained earlier, identifying mutation/variation of genes involved in the type I IFN pathway should be evaluated for potential roles in the familial form of PD. Additionally, an understanding of how the type I IFN pathway contributes to neuronal homeostasis, and survival and how its dysregulation causes neuronal cell death / neurodegeneration yet still need to be defined.
The paper
PIAS2-mediated blockade of IFN-β signaling: A basis for sporadic Parkinson disease dementia
Joana Magalhaes, Emilie Tresse, Patrick Ejlerskov, Erling Hu, Yawei Liu, Andrea Marin, Alexia Montalant, Letizia Satriano, Carsten Friis Rundsten, Eva Maria Meier Carlsen, Rasmus Rydbirk, Ali Sharifi-Zarchi, Jesper Bøje Andersen, Susana Aznar, Tomasz Brudek, Konstantin Khodosevich, Marco Prinz, Jean-François Marie Perrier, Manu Sharma, Thomas Gasser, and Shohreh Issazadeh-Navikas.
Molecular Psychiatry, July 2021
First shared co-author
 Emilie Tresse-Gommeaux is Assistant Professor in Issazadeh-Navikas Group. Her research focuses on the role of mitochondrial dysfunction on neuroinflammation and neurodegeneration.
Emilie Tresse-Gommeaux is Assistant Professor in Issazadeh-Navikas Group. Her research focuses on the role of mitochondrial dysfunction on neuroinflammation and neurodegeneration.
