FEATURE - Unraveling the mysteries of a rare cancer
February 12th is World Cholangiocarcinoma Day - an international effort to raise awareness of the rare and highly aggressive cancer of the bile ducts. Amongst the researchers behind the initiative is Group Leader Jesper B. Andersen from Biotech Research & Innovation Centre (BRIC) . The Andersen group has for years been focused on utilizing large clinical and molecular datasets to extract new knowledge about the molecular mechanisms of this disease.
Cholangiocarcinoma (CCA) is a rare cancer type that forms in the bile ducts. These ducts are slender tubes that carry the digestive fluid - bile - from the liver to the gallbladder and small intestine. In Denmark, approx. 250 patients are diagnosed with this cancer form each year, and the average survival is only 12 months from the time of diagnosis. The survival rate has not improved since the 1980s and there are currently no approved treatments available in DK. In Western countries, the disease is associated with modern lifestyle-associated risk factors such as alcohol consumption, diabetes and obesity and the incidence of CCA has more than doubled during the last 30 years.
Mapping the molecular landscape of disease
The quest for a “one-size-fits-all” solution to manage cancer has long been abandoned as useless, since patients who share a common cancer diagnosis can have tumors that behave very differently. In the Andersen Group, researchers aim to divide patients with CCA into subgroups, in which the tumors behave more similarly. By doing so, the researchers hope to develop molecular tools to identify the patients earlier in their disease progression, to understand the genetic changes in these subgroups and in the future develop tailored therapies for these patients. In order to identify disease patterns, the group uses modern sequencing techniques to investigate large sets of so-called omics data:
Omics is a joint term for a series of biological markers or molecules that all share the suffix -omics. (genomics, proteomics, metabolomics etc.). Together, these molecules translate into the structure, function and dynamics of our bodies, and through mass omics sequencing, researchers characterize and quantify different groups of the molecules. Through these methods, researchers can create individual patient molecular profiles. By comparing large amounts of data, the idea is to locate patterns, which enables them to divide patients into subtypes to facilitate precision therapy. Alternatively, uncovering key changes in the molecular profiles of patients can be used as diagnostic and prognostic biomarkers.
Identifying patient subgroups
In 2017 researchers from the Andersen group analyzed genome-wide data obtained from 496 intrahepatic cholangiocarcinoma (iCCA) patients and identified three subtypes of iCCA patients based on the mutational status of three individual classifier genes (IDH, KRAS, TP53). Each patient subtype was characterized by a specific molecular pattern and in order to test the clinical implications, the researchers screened a library of 525 drugs in patient-matched cell models. The results showed that the different mutational profiles influenced a precise response to treatment. Read more here:
https://www.bric.ku.dk/research-stories/unique-oncogenetic-programmes-influence-response-to-drug-treatment-in-bile-duct-cancer-patients/. The study is an example of a so-called drug repositioning, in which researchers investigates existing drugs for new therapeutic purposes.
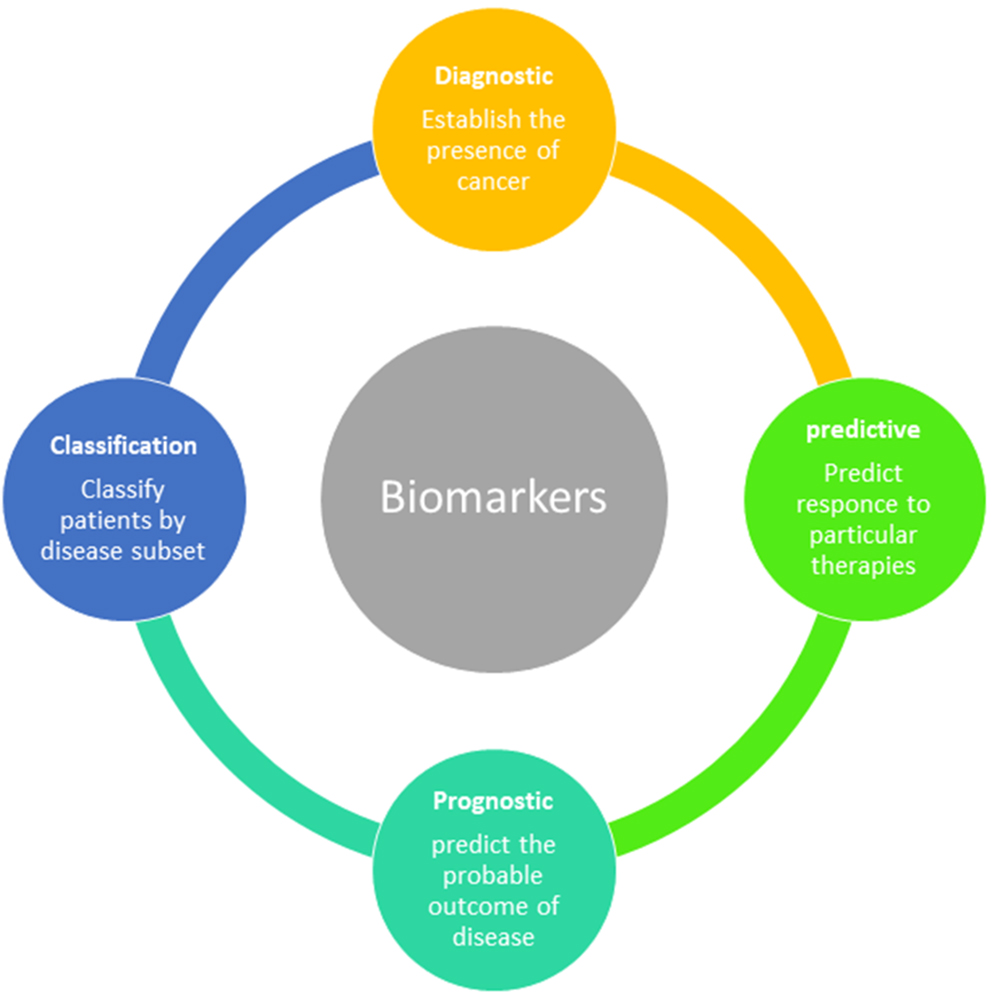
Biomarkers – more than diagnostic tools
One of the main aims of Andersen Group is to identify biomarkers. In 2020, researchers in the Andersen group collaborated with investigators at Herlev and Gentofte Hospital in identifying a biomarker that reliably predicts how aggressive a patient’s disease will evolve. A prognostic biomarker may in the future help doctors in the hospitals to make the right decisions about the benefit of chemotherapy for each CCA patient. The study is an example of how the discovery of molecular tools or “biomarkers” can help clinicians decide which patients to treat, what treatments to use, when treatments are actively working and when patients stop benefiting from the therapy.
The researchers measured the levels of two specific proteins, and a biomarker (CA19-9) currently used in the clinic, in the blood (so called liquid biopsies) before and during chemotherapy in patients with advanced disease, and found that patients with higher levels of the new biomarkers before chemotherapy had a lower survival rate. Read more about the results here https://www.bric.ku.dk/research-stories/research-stories-2020/biomarker-reveals-how-aggressive-biliary-tract-cancer-is-in-patients/
A common misperception may be that biomarkers are mainly needed to diagnose a specific cancer type (diagnostic markers), but diverse biomarkers are also needed to guide clinical decision-making throughout each patients’ individual journey. These types of prognostic and predictive biomarkers deserve increased attention, in particular as they are playing important roles in the increasingly individualized management of more common cancer types, Jesper B. Andersen
Challenges and opportunities
While recent year’s technological advancements have brought new opportunities there are still many challenges, the biggest of which is to find sufficient patient material to secure statistically significant results. However, stronger international collaborations between researchers have come a long way to overcome these challenges. In spring 2020 the U.S. Food and Drug Administration (FDA) approved the first ever targeted treatment for a subgroup of this disease. The drug, which works by inhibiting a protein from a so-called fusion gene, is not yet approved in Europe.
Jesper B. Andersen also notes a rising awareness and focus on research in rare cancers. In 2020, the Danish Cancer Society made an open call specifically for studies of rare cancers and through this call Jesper B. Andersen’s research group was awarded 1.6 M DKK for a new research project:
- Through our research, we have shown that approx. 50% of patients with cancer in the bile ducts have damages on genes that function by regulating a process that chemically modifies other genes and their function (epigenetic changes). When these genes no longer can be read correctly the growth and spread of cancer increase. In our new project, we investigate how this process leads to the formation of metastasis and if certain proteins play a particularly important role in regard to metastatic process. It is therefore important that patients with CCA are offered genomic tests. In the longer run, we may be able to use this knowledge to develop new precision therapies. Jesper B Andersen, Group leader
Read more:
Visit the Andersen Lab website here
Follow Andersen Lab on twitter
Read more about world cholangiocarcinoma day here
