PUBLICATION SPOTLIGHT - The long non-coding RNA MIR31HG regulates the senescence associated secretory phenotype
By Marta Montes Resano, Assistant professor, Lund Group
How would you summarise your study in one sentence?
The long non-coding RNA (lncRNA) MIR31HG regulates the expression and secretion of a subset of components from BRAF-induced senescent cells through interaction with YBX1 to promote translation of IL1A.
Can you describe briefly what you have explored and how?
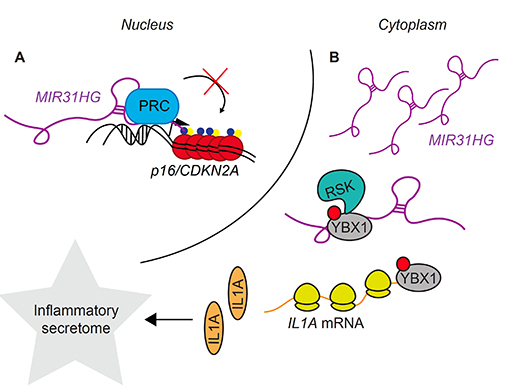
BRAF is often activated by oncogenic mutations in cancers such as melanoma. In this study, we focus on the senescence process initiated following the expression of a constitutively active BRAF. We have performed functional studies, in vitro analysis and biochemistry approaches to decipher how the cytoplasmic lncRNA MIR31HG reduces the secretion of inflammatory components by senescent cells that can impact the neighboring cells and tissues. The secretome from BRAF-senescent cells lacking MIR31HG is depleted for several inflammatory factors, including IL6 and CXCL1, and in consequence fails to induce invasion. Mechanistically, this occurs through modulating the translation of IL1A, an upstream regulator of the SASP. We have demonstrated that during BRAF-induced senescence MIR31HG interacts with the translation factor YBX1, promotes its interaction with the RSK kinase, which facilitates YBX1 phosphorylation and in turn, favors IL1A translation.
What would you say is the novelty in this study?
Our results provide a new lncRNA-mediated regulatory mechanism, highlighting the role of lncRNAs as regulators of critical processes such as oncogene-induced senescence. Excitingly, our results suggest a novel role for MIR31HG in senescence induced by oncogenes depending on the regulation of the pro-inflammatory branch of the secretome. Considering our previous findings (Montes M et al. Nat Comm 2015), we have uncovered a dual role for the lncRNA MIR31HG in regulating senescence depending on its localization. In young proliferating cells nuclear lncRNA MIR31HG interacts with polycomb repressor complexes to inhibits the expression of the tumor suppressor and key regulator of senescence, p16/CDKN2A.During BRAF-induced senescence MIR31HG is up-regulated, released from chromatin allowing p16 expression and translocates to the cytoplasm where we now show it has a role in the regulation of expression of the inflammatory components of the senescence secretome.
How do the results relate to the scientific field with respect to what is already known?
Although lncRNAs have recently emerged as major players in fundamental biological processes, the functionality and the molecular mechanism of many of these transcripts remains to be elucidated. Here, we are uncovering the molecular mechanism of MIR31HG in the regulation of secreted proteins during BRAF-induced senescence.
Senescence induced by oncogenes has been considered a mechanism to suppress malignant transformation due to the inhibition of cellular proliferation. However, it can also promote cancer through the secretion of pro-inflammatory factors released from senescent cells onto the neighbouring tissues. These opposite effects are mediated by the SASP, the regulation of which is very complex and remains to be fully characterized. Therefore, we are contributing knowledge to expand our understanding of the regulation of the SASP, which is crucial to be able to fully understand the physiological role of senescence and potentially harness this knowledge for therapeutic purposes.
Can you describe the potential of the findings beyond the research field?
Somatic mutations in BRAF occur in approximately 7% of human cancer. The fact that depletion of MIR31HG decreases pro-invasive factors during SASP induction upon BRAF activation, makes it an interesting target for therapies. Inhibition of MIR31HG as well as other anti-SASP therapies could be potentially used to ameliorate the detrimental effects of senescence cells in cancer and other aging-related pathologies. Many treatments that target or deliver RNA are in the development pipeline or being tested in clinical trials. The success of RNA-therapeutics and the innovative technologies that are currently under development, makes lncRNAs feasible targets for clinical purposes.
What are the next stages in this research?
The next step would be to investigate the therapeutic potential of depleting MIR31HG in vivo. We would use sophisticated mice models carrying cancer patient-derived cells to try to address the role of this lncRNA in tumor progression in vivo.
The paper
"The long non-coding RNA MIR31HG regulates the senescence associated secretory phenotype"
Marta Montes1*, Michal Lubas1, Frederic S Arendrup1, Bettina Mentz1, Neha Rohatgi2, Sarunas Tumas1, Lea M Harder3, Anders J Skanderup2, Jens S Andersen3, Anders H Lund1*
1Biotech Research and Innovation Centre, University of Copenhagen, Denmark.2 Genome Institute of Singapore, Agency for Science, Technology and Research (A*STAR), Singapore. 3 Department of Biochemistry and Molecular Biology, University of Southern Denmark, Odense, Denmark. *Corresponding authors
Nature Communications, April 2021
First Author
 Marta Montes is an Assistant Professor in the Lund Group at the Biotech Research & Innovation Centre
Marta Montes is an Assistant Professor in the Lund Group at the Biotech Research & Innovation Centre
Her research has been focused on studying the molecular mechanism of lncRNAs in the regulation of oncogene-induced senescence and cancer.
