Engelholm Group
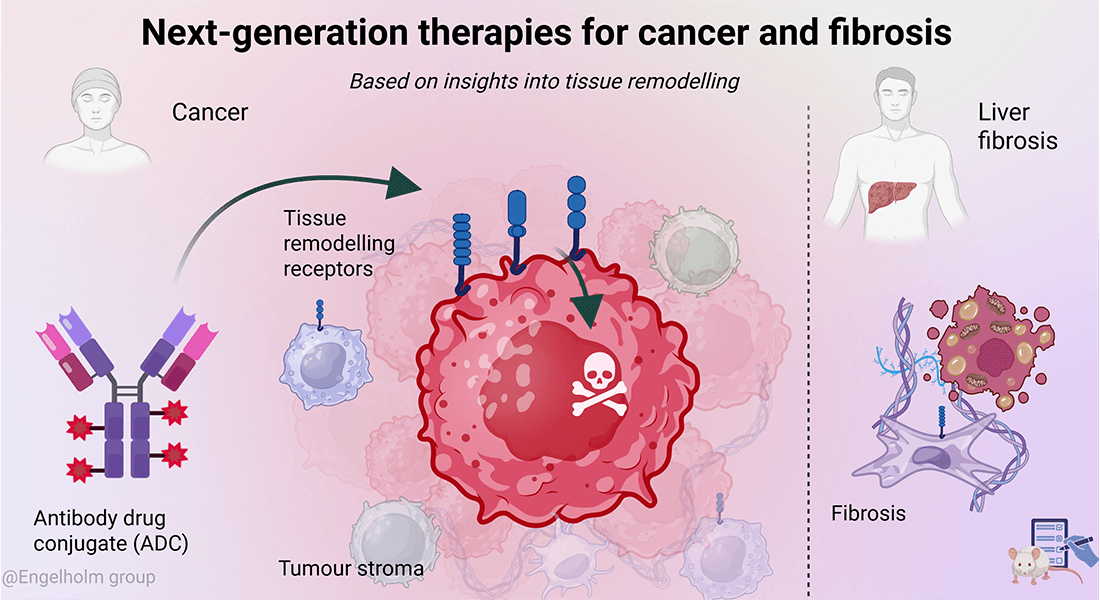
The Engelholm Group is pioneering next-generation therapies for cancer and fibrosis through innovative targeting strategies.
The group initially focused on uncovering key cellular receptors and signalling pathways involved in pathological tissue remodelling. Building on this foundation, our research has transitioned toward a more translational approach.
Using a variety of in vivo models and molecular techniques, we now explore the therapeutic potential of targeting these receptors, primarily through the development of antibody-drug conjugates (ADCs).
We span the full ADC development pipeline, from target discovery and antibody engineering to ADC design and functional testing in both in vitro and in vivo models.
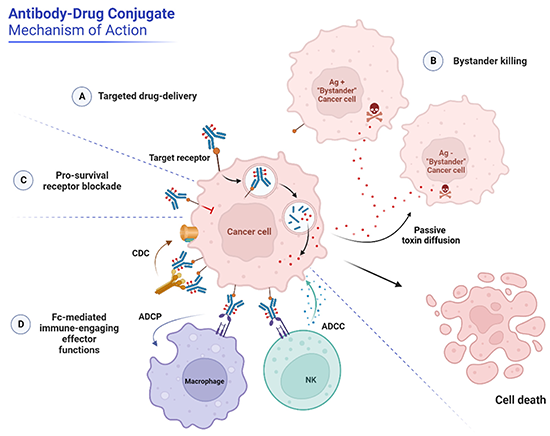
Antibody-drug conjugates (ADCs) eliminate tumor cells through several mechanisms. Their primary mode of action is targeted drug delivery, where the toxic payload is released inside cancer cells after ADC internalization. In addition, some ADCs can affect neighboring cells through a "bystander effect" or engage the immune system to enhance antitumor activity. Image from Metrangolo, V., & Engelholm, L. H. (2024). Antibody-Drug Conjugates: The Dynamic Evolution from Conventional to Next-Generation Constructs. 1–20.
Our vision is to transform fundamental scientific discoveries into clinically meaningful solutions.
We aim to develop effective, targeted therapies for various cancer indications, bridging the gap between basic research and real-world treatment to ultimately enhance outcomes for patients.
Please note that the research conducted in this group is still in its early stages and not yet ready for human use. Individuals seeking investigational therapies are encouraged to consult a healthcare provider or explore clinical trial registries for appropriate guidance and options.
uPARAP/Endo180 as an ADC target
We have studied the uPARAP/Endo180 collagen-degrading receptor. This receptor does not only regulate extracellular matrix turnover but also drives bone destruction in osteosarcoma. Its cancer-enriched expression and role in pathological remodeling make it a high-priority target for therapeutic development, including antibody‑drug conjugate strategies.
- Engelholm LH, Ingvarsen S, Jürgensen HJ, Hillig T, Madsen DH, Nielsen BS, Behrendt N. The collagen receptor uPARAP/Endo180. Front Biosci (Landmark Ed). 2009 Jan 1;14(6):2103-14. doi: 10.2741/3365. PMID: 19273187.
- Jürgensen HJ, Madsen DH, Ingvarsen S, Melander MC, Gårdsvoll H, Patthy L, Engelholm LH, Behrendt N. A novel functional role of collagen glycosylation: interaction with the endocytic collagen receptor uparap/ENDO180. J Biol Chem. 2011 Sep 16;286(37):32736-48. doi: 10.1074/jbc.M111.266692. Epub 2011 Jul 18. PMID: 21768090; PMCID: PMC3173195.
- Engelholm LH, Melander MC, Hald A, Persson M, Madsen DH, Jürgensen HJ, Johansson K, Nielsen C, Nørregaard KS, Ingvarsen SZ, Kjaer A, Trovik CS, Laerum OD, Bugge TH, Eide J, Behrendt N. Targeting a novel bone degradation pathway in primary bone cancer by inactivation of the collagen receptor uPARAP/Endo180. J Pathol. 2016 Jan;238(1):120-33. doi: 10.1002/path.4661. Epub 2015 Nov 30. PMID: 26466547.
Professor Lars Engelholm played an important role in the scientific foundation of Adcendo. The pioneering research on uPARAP/Endo180 carried in the group helped establish it as a promising target for cancer therapy, laying the groundwork for Adcendo’s lead ADC program.
Physiological role of uPARAP/Endo180
We have shown that uPARAP/Endo180 is a structurally complex collagen receptor whose function relies on cooperative inter-domain interactions.
It mediates collagen endocytosis in both fibroblasts and some macrophages, facilitates lymphatic vessel growth, and helps regulate immune functions through the uptake of collectins.
Its multifunctional roles in both physiological repair and pathological remodeling make it a compelling therapeutic target, supporting our translational focus on ADC-based strategies targeting uPARAP/Endo180.
- Jürgensen HJ, Johansson K, Madsen DH, Porse A, Melander MC, Sørensen KR, Nielsen C, Bugge TH, Behrendt N, Engelholm LH. Complex determinants in specific members of the mannose receptor family govern collagen endocytosis. J Biol Chem. 2014 Mar 14;289(11):7935-47.
- Jürgensen HJ, Silva LM, Krigslund O, van Putten S, Madsen DH, Behrendt N, Engelholm LH, Bugge TH. CCL2/MCP-1 signaling drives extracellular matrix turnover by diverse macrophage subsets. Matrix Biol Plus. 2019 Mar 19; 1:100003.
- Jürgensen HJ, van Putten S, Nørregaard KS, Bugge TH, Engelholm LH, Behrendt N, Madsen DH. Cellular uptake of collagens and implications for immune cell regulation in disease. Cell Mol Life Sci. 2020 Aug;77(16):3161-3176.
uPAR as an ADC target
We study the urokinase plasminogen activator receptor (uPAR) as a promising ADC target in stroma-rich cancers like pancreatic ductal adenocarcinoma (PDAC) and demonstrates that FL1-PNU not only directly kills tumour cells but also fosters a more favourable immune landscape, paving the way for future clinical development of uPAR-targeting ADCs.
- Metrangolo, V., Hansen Blomquist, M., Dutta, A., Gårdsvoll, H., Krigslund, O., Sandal Nørregaard, K., Jessen Jürgensen, H., Ploug, M., Flick, M. J., Behrendt, N., & Engelholm, L. H. (2025). Targeting uPAR with an antibody-drug conjugate suppresses tumor growth and reshapes the immune landscape in pancreatic cancer models. In Sci. Adv (Vol. 11).
- Metrangolo V, Ploug M, Engelholm LH. The Urokinase Receptor (uPAR) as a "Trojan Horse" in Targeted Cancer Therapy: Challenges and Opportunities. Cancers (Basel). 2021 Oct 27;13(21):5376. doi: 10.3390/cancers13215376. PMID: 34771541; PMCID: PMC8582577.
Our current research focuses on the preclinical development of targeted therapies for cancer.
Key projects include:
- Advancing our anti-uPAR antibody–drug conjugate (ADC) through in vivo efficacy testing and translational studies.
- Development of an anti–MT1-MMP ADC for hard-to-treat cancers.
- Development of an anti-Mannose Receptor ADC targeting the tumor microenvironment.
- Investigating the role of key cell types and pathways in liver fibrosis, with a focus on cellular crosstalk and microenvironmental changes in both fibrotic and cancerous conditions.
Our group applies a comprehensive technological platform to support the development of next-generation antibody–drug conjugates (ADCs).
This includes state-of-the-art molecular engineering of the antibody backbone, careful selection of relevant cytotoxic payloads, and advanced ADC production.
The performance of each ADC is thoroughly assessed through various in vitro assays, followed by extensive preclinical testing in vivo to evaluate its safety and therapeutic potential. This is performed in orthotopic xenograft mouse models for various cancers.
We employ both genetically modified (GMO) and non-modified (non-GMO) mouse models to test ADC efficacy, explore novel ADC targets, and investigate fibrotic mechanisms in cancer and other fibrotic diseases.
22 January 2025
Potential Pancreatic Cancer Treatment Shows Promise
20 January 2025
Forskning kan bane vejen for ny behandling af bugspytkirtelkræft (Danish)
24 October 2024
The Antibody Society: Interview with Virginia Metrangolo (University of Copenhagen) - YouTube
30 August 2024
Millionbevilling fra Lundbeckfonden til KU- og DTU-forsker (Danish)
8 July 2024
På vej med mere effektivt tumor-drab (Danish)
2 June 2023
Forskere vil udvikle nyt lægemiddel mod aggressive kræftformer (Danish)
29 April 2021
Kæmpe investering til trojansk angreb på kræftceller (Danish)
Katrine Qvortrup, DTU, DK
Brita Singers Sørensen, AU, DK
Matthias Manfred Herth, UCPH, DK
Andreas Kjær, UCPH, DK
Michael Ploug, UCPH, DK
Eric Santoni-Rugiu, UCPH, DK
Matthew J. Flick, UNC, US
Bo Torben Porse, UCPH, DK
Inna Chen, HGH, DK
Rikke Eefsen, HGH, DK
Lundbeckfonden
Kræftens Bekæmpelse
Novo Nordisk Foundation
Innovationsfonden
Spark Denmark
Marie Curie Actions
Neye
BioInnovation Institute
Find Fonden





