Light Microscopy
The BRIC Light Microscope Facility offers access to two confocal microscopes, Leica SP8 and one Zeiss LSM 800 confocal fluorescence microscopes, two high through put microscopes, Olympus ScanR, and two widefield Zeiss AxioImager microscopes.
Microscopes are available 24/7 for internal users while external users can have access during normal business hour.
Support, including mandatory training on systems, can be booked by emailing staff scientist Yasuko Antoku.
The facility offers service based support
- Training: All users of core microscopes need mandatory training in order to book and use core microscopes.
- Support: Advice users on choosing specific microscopes for specific projects, assisting users with acquiring fluorescence images and analysis for publication and provide either full support or partial support during microscope sessions.
- Analysis of fluorescence images: Quantification/co-localization analysis.
Users can have access to the following booking site after mandatory training from staff scientist Yasuko Antoku on systems: PPMS for the Microscopy and Histology
Prices for internal UCPH users
| Confocal Microscope Leica Confocal sp8 | Usage 100 DKK/hour |
| Confocal Microscope Zeiss Confocal LSM800 | Usage 100 DKK/hour |
| Olympus ScanR Anja | Usage 50 DKK/hour |
| Olympus ScanR Kim | Usage 50 DKK/hour |
| Upright fluorescence microscope Zeiss AxioImager M2 | Usage 50 DKK/hour |
| Inverted Olympus microscope (3.4.24) | Usage Free |
| DeltaVision Elite | Usage 100 DKK/hour |
| Upright histological Olympus microscope (3.4.24) | Usage Free |
| Image processing workstation (1.3.24) | Usage Free |
| Training and Personal support | 400 DKK/hour |
Image processing workstation
Free Software
Microscopes

Description
Multiplexing inverted widefield microscope from Miltenyi Biotec and designed for fully automated cyclic staining and imaging of hundreds of markers on a single sample or multiple samples, and data analysis of biological samples with automatic XY stage, motorized focus, and hardware autofocusing.
|
Fluorescence light source |
LED illumination system |
|---|---|
|
Objectives |
2× objective to generate overview images; NA 0.1 20× long working distance objective (designed for 1 mm thick slides); NA 0.45 20× objective with high numerical aperture (designed for 170 μm thick cover glass); NA 0.75 |
|
Excitation |
Six excitation LEDs (filters: 386/23 nm, 420/10 nm, 470/40 nm, 531/46 nm, 628/32 nm, 725/40 nm) |
|
Emission |
Five emission filters (470/40 nm, 530/43 nm, 580/25 nm, 698/70 nm, 809/81 nm) |
|
Camera |
sCMOS camera (15 megapixel, 25 mm diagonal field of view) |
|
MACS Software |
MACSima™ (Acquisition); MACS iQ View (analysis) |
|
Liquid handling system
|
Robotic needle arm allowing fully automated liquid transfer
|
|
MACS staining method |
Cyclic Staining (DAPI/FITC/PE/APC) |
|
Autofocus |
Dual approach of hardware- and image-based autofocus mechanisms |
|
Bleaching unit |
Illuminated area: 3 mm × 3 mm Light intensity: 2 W |
|
Location |
3.4.50A |
|
Format |
|
|
Reference |
Spatial biology | MACSima™ Platform | Multiplex Imaging | Miltenyi Biotec | Deutschland |

Description
ZEISS Laser Confocal Scanning Microscope with super-resolution Airyscan. Incubation chamber for live imaging with controlled CO2 and temperature. Microscope is equipped with four solid state lasers, a GaAsP PMT detectors and one T-PMT detector for transmission light detection.
|
Lasers |
405, 488, 561, 640 nm |
|---|---|
|
Detectors |
1 x GaAsP PMT, 1 x T- PMT detector |
|
Objectives |
Plan-Apochromat 10x / 0.45 AIR, Plan-Apochromat 25x / 0.80 IMM Plan-Apochromat 40x / 1.3 OIL, C-Apochromat 40x / 1.2 W, Plan-Apochromat 63x / 1.4 OIL. |
|
Fluorescence light source |
HXP 120V |
|
Filters for fluorescence |
|
|
Transmitted light techniques |
Brightfield |
|
Software |
ZEN Blue |
|
Location |
3.3.14 |
|
Reference |
Description
Inverted microscope Leica SP8 is an automated inverted confocal laser scanning microscope with a very flexible white light laser source and super-sensitive Hybrid detectors (HyD).
|
Lasers |
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Detectors |
2 x super-sensitive Hybrid detectors (HyD), 1 x T- PMT detector |
|||||||||||||||||
|
Objectives |
HC Plan-Apochromat 10x / 0.40 AIR, HC Plan-Apochromat 20x / 0.75 IMM, HC Plan-Apochromat 40x / 1.1 W, HC Plan-Apochromat 63x / 1.40 OIL. |
|||||||||||||||||
|
Fluorescence light source |
HXP 120V |
|||||||||||||||||
|
Filters for fluorescence |
|
|||||||||||||||||
|
Transmitted light techniques |
Brightfield |
|||||||||||||||||
|
Software |
LASX |
|||||||||||||||||
|
Location |
4.3.26 |
|||||||||||||||||
|
Reference |
||||||||||||||||||

Description
Widefield upright microscope Zeiss AxioImager M2
|
Fluorescence light source |
HXP 120V |
|---|---|
|
Filters for fluorescence |
|
|
Cameras |
Hamamatsu ORCA R2 |
|
Objectives |
ECPlan-Neofluar 10x / 0.30 AIR, ECPlan-Neofluar 20x / 0.5 AIR, ECPlan-Neofluar 40x / 0.75 AIR, Plan-Apochromat 63x / 1.4 OIL, Plan-Apochromat 100x / 1.40 OIL |
|
Transmitted light techniques |
Brightfield, Differential interference contrast, Phase contrast, Darkfield |
|
Software |
ZEN Blue |
|
Location |
3.4.44 |
|
Reference |

Description
Widefield upright microscope Zeiss AxioImager M2
|
Fluorescence light source |
HXP 120V |
|---|---|
|
Filters for fluorescence |
|
|
Cameras |
AxioCam MR2 |
|
Objectives |
N Plan-Apochromat 10x / 0.25 AIR, ECPlan-Neofluar 20x / 0.5 AIR, ECPlan-Neofluar 40x / 0.75 AIR, Plan-Apochromat 63x / 1.4 OIL, Plan-Apochromat 100x / 1.40 OIL |
|
Transmitted light techniques |
Brightfield, Differential interference contrast, Phase contrast, Darkfield |
|
Software |
ZEN Blue |
|
Location |
3.4.44 |
|
Reference |

Description
High-throughput content screening (HCS) inverted widefield microscope from Olympus and designed for fully automated image acquisition and data analysis of biological samples with automatic XY stage, motorized focus, and hardware autofocusing, and incubation chamber for live imaging with controlled CO2 and temperature.
|
Fluorescence light source |
High power LED illumination system: Lumencor SpectraX light engine |
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Objectives |
UPlanSApo 4x / 0.16 AIR, PlanC N 10x/ 0.25 AIR, UPlanSApo 20x / 0.75 AIR UPlanSApo 40x / 0.95 AIR, LUCPlan-FLN 60x / 0.7 AIR. |
||||||||||||||||||||||||||||
|
Filters for fluorescence |
|
||||||||||||||||||||||||||||
|
Transmitted light techniques |
Brightfield |
||||||||||||||||||||||||||||
|
Camera |
Hamamatsu ORCA-FLASH 4.0, high-sensitivity digital monochrome scientific cooled sCMOS camera |
||||||||||||||||||||||||||||
|
Software |
ScanR Acquisition and Analysis V2.8 |
||||||||||||||||||||||||||||
|
format |
Well plate and slides |
||||||||||||||||||||||||||||
|
Location |
3.4.16 |
||||||||||||||||||||||||||||
|
Reference |

Description
High-throughput content screening (HCS) inverted widefield microscope from Olympus and designed for fully automated image acquisition and data analysis of biological samples with automatic XY stage, motorized focus, and hardware autofocusing.
|
Fluorescence light source |
High power LED illumination system: Lumencor SpectraX light engine |
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Objectives |
UPlaFl 10x/ 0.3 AIR, UPlanSApo 20x / 0.75 AIR UPlanSApo 40x / 0.95 AIR. |
||||||||||||||||||||||||||||
|
Filters for fluorescence |
|
||||||||||||||||||||||||||||
|
Transmitted light techniques |
Brightfield |
||||||||||||||||||||||||||||
|
Camera |
Hamamatsu ORCA-FLASH 4.0, high-sensitivity digital monochrome scientific cooled sCMOS camera |
||||||||||||||||||||||||||||
|
Software |
ScanR Acquisition and Analysis V2.8 |
||||||||||||||||||||||||||||
|
format |
Well plate and slides |
||||||||||||||||||||||||||||
|
Location |
4.3.12 |
||||||||||||||||||||||||||||
|
Reference |
Modular High-Content Screening Station for Life Sciences |
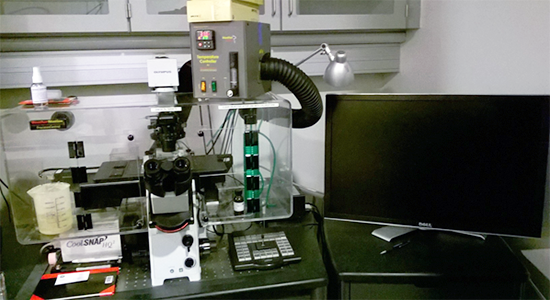
Description
Inverted decovolution fluorescence widefield microscope with XYZ motorized stage/focus control, environmental control for live cell imaging, time lapse, multi point, and tile scanning from Olympus.
| Fluorescence light source | High power LED illumination system: Lumencor SpectraX light engine (https://lumencor.com/products/spectra-x-light-engine/) | ||||||||||||||||||||||||||||
| Objectives | UPlanSApo 10x/ 0.4 AIR, LCPlanFl 20x / 0.4 AIR, UApo/340 40x / 1.35 Oil, PlanApoN 60x/ 1.42 OIL, UPlanSApo 100x/ 1.4 OIL. | ||||||||||||||||||||||||||||
| Filters for fluorescence |
|
||||||||||||||||||||||||||||
| Transmitted light techniques | Brightfield and DIC | ||||||||||||||||||||||||||||
| Camera | Photometrics CoolSNAP HQ camera | ||||||||||||||||||||||||||||
| Software | SoftWoRx V 7.2.2 | ||||||||||||||||||||||||||||
| Format | 4.3.12 | ||||||||||||||||||||||||||||
| Reference | https://www.imsol.co.uk/deltavision-elite/ | ||||||||||||||||||||||||||||

Description
Fully-automated, inverted, multi-channel fluorescence and transmitted light imaging system coupled with capabilities for multi-position well scanning, Z-stack, tile-stitching, live-cell imaging, time lapse, and autofocus from Thermofisher Scientific.
| Fluorescence light source | LED light sources |
| Objectives | 4X (AMEP4680), 10X (AMEP4681), 20X (AMEP4982), 40X (AMEP4683 and AMEP4754) |
| Filters for fluorescence |
GFP Ex 482/25, Em 524/24 RFP Ex 542/20, Em 593/40 |
| Transmitted light techniques | Brightfield |
| Camera | Colour and Monochrome camera |
| Software | Auto 2 |
| Format | Wellplate and slides |
| Location | 3.4.10 |
| Reference | https://www.thermofisher.com/dk/en/home/life-science/cell-analysis/cellular-imaging/evos-cell-imaging-systems.html |
Contact

Yasuko Antoku, staff scientist
Yasuko.Antoku@bric.ku.dk
BRIC - Biotech Research & Innovation Centre
Ole Maaløes Vej 5
DK-2200 Copenhagen
Room 3-4-37
